gas volume analysis|measure volume of hydrogen gas : trade Definitions. A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. Consider k gases in a rigid container as shown here. The properties of the .
weblyrics aud-20231027-wa0001 : baixar jogos de cassino lyrics aud-20231027-wa0001 16 de abr. de 2019 · Couv. art. (1º sáb. do mês) R$ 44. Horários. .
{plog:ftitle_list}
WEB24 de dez. de 2021 · Looking for information on the manga Dreaming Freedom? Find out more with MyAnimeList, the world's most active online anime and manga community .
The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. The gas forces water out of the container, and the volume of liquid displaced is a measure of the volume of gas.
The molar volume of a gas is the volume of one mole of a gas at STP. At STP, one .
This ideal gas volume calculator finds the volume of an ideal gas given the amount of gas and its temperature. We'll explain in this short article: What an ideal gas is; How to calculate its volume using the ideal gas law; . The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles’s law). The volume of a given amount of gas is .The volume (\(V\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (P) and the number of temperature (T) are constant. We can express this .Definitions. A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. Consider k gases in a rigid container as shown here. The properties of the .
natural gas volume measurement
In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of multicomponent gas mixtures, which are .
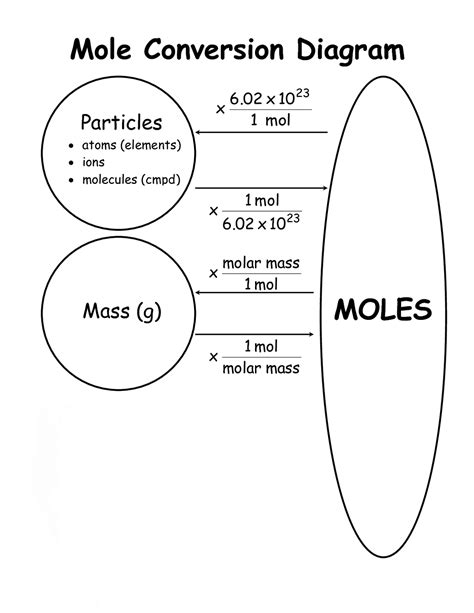
The molar volume of a gas is the volume of one mole of a gas at STP. At STP, one mole (\( 6.02 \times 10^{23}\) representative particles) of any gas occupies a volume of \(22.4 .Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. Use Avogadro’s number to convert between .
Due to the interaction of hydrocarbon molecules, hydrocarbon gases deviate from the Ideal Gas Law. By analyzing the composition of natural gas, one can adjust the Ideal Gas Law to . Gas chromatography is a term used to describe the group of analytical separation techniques used to analyze volatile substances in the gas phase. . A calibrated microsyringe is used to deliver a sample volume in the .
moles per standard cubic foot
Based on the Ideal Gas Law, one can derive a very important relationship. If a gas is composed of different types of molecules (i.e., nitrogen, oxygen, etc.), the volume percentage of each gas is equal to the molar percentage of that gas. This can be shown as follows: Let V be the total gas volume and N be the total number of moles. The Ideal .
Blood gas analysis is a commonly used diagnostic tool to evaluate the partial pressures of gas in blood and acid-base content. Understanding and using blood gas analysis enables providers to interpret respiratory, circulatory, and metabolic disorders.[1]Combustion analysis involves the measurement of gas concentrations, temperatures and pressure for boiler tune-ups, emissions checks and safety improvements. Parameters that are commonly examined . % Gas Concentration by Volume 60 80 100 120 140 160 180 16.0 14.0 12.0 10.0 8.0 6.0 4.0 2.0 0.0 . Combustion Analysis Basics . To better understand reserves estimation, a few important terms require definition. Original oil in place (OOIP) and original gas in place (OGIP) refer to the total volume of hydrocarbon stored in a reservoir prior to production.Reserves or recoverable reserves are the volume of hydrocarbons that can be profitably extracted from a reservoir using existing .8 Example pressure profile for small-scale gas analysis tests 11 9 Gas volume emitted from 18650 LiCoO. 2. cell 13 10 Major gas species concentrations for LiCoO. 2. 18650 cells 14 11 Individual gas species concentrations for LiCoO. 2. 18650 cells 15 12 Flammable gases emitted from LiCoO2 18650 cells 16
measure volume of hydrogen gas
Combustion analysis is a standard method of determining a chemical formula of a substance that contains hydrogen and carbon. . using a multiple-flame alcohol lamp (Figure \(\PageIndex{5}\)) and measuring the change in gaseous volume. Figure \(\PageIndex{4 . low NO x stationary gas turbine: Premixed: Laminar: Flat flame, Bunsen flame . In conventional core analysis, gas volume is determined on one sample while oil and water volumes are measured on a second sample. Significant variations in pore system quality between samples can cause errors in fluid saturation and porosity values.
Volume of a Gas Sample The sample of gas in has a volume of 15.0 mL at a pressure of 13.0 psi. Determine the pressure of the gas at a volume of 7.5 mL, using: (a) the P–V graph in (b) the \(\frac{1}{P}\) vs. V graph in (c) the Boyle’s law equation. Comment on the likely accuracy of each method. SolutionExample: pressure-volume and shaft work¶ Consider a system with pressure-volume work from a piston and shaft work. We should choose the control surface appropriately so that there is no fluid motion at the boundary, except where. fluid enters and leaves the control volume, and. a device (e.g., shaft) crosses the boundary.
Stoichiometric or Theoretical Combustion is the ideal combustion process where fuel is burned completely. A complete combustion is a process burning all the carbon (C) to (CO 2), all the hydrogen (H) to (H 2 O) and all the sulphur (S) to (SO 2).. With unburned components in the exhaust gas such as C, H 2, CO, the combustion process is uncompleted and not .
An Arterial Blood Gas requires the nurse to collect a small sample of blood - generally, a full 1 ml³ is preferred. Blood can be drawn via an arterial stick from the wrist, groin, or above the elbow. . Once the blood is obtained, it is either sent to the hospital’s central lab for analysis or tested by the respiratory therapist on the unit . The sorption analysis indicates that the larger free volume provides more sites for gas molecule absorption. The MD and MC results provide good agreement with the experimental data from past reports, which validates the feasibility of molecular simulation techniques in gas separation membranes at a molecular scale.Applying the Ideal Gas Law. The ideal gas law allows us to calculate the value of the fourth variable for a gaseous sample if we know the values of any three of the four variables (P, V, T, and n).It also allows us to predict the final state of a sample of a gas (i.e., its final temperature, pressure, volume, and amount) following any changes in conditions if the parameters (P, V, T, .in swelling gas are some permanent gases and light hydrocarbons. Gas chromatography is usually used for LIBs swelling gas analysis. However, some small-size LIBs can only generate several milliliters of swelling gases during their service. This gas volume is not adequate for the effective purge of the sample-load flow path in the gas
This paper presents an integrated study from fracture propagation modeling to gas flow modeling and a correlation analysis to explore the key controlling factors of intensive volume fracturing. The fracture propagation model takes into account the interaction between hydraulic fracture and natural fracture by means of the displacement discontinuity method (DDM) and .Analysis. Read off the volume of gas produced for a sensible mass of sodium carbonate, e.g. 0.35 g produces 79.0 cm 3 The mass of sodium carbonate may be specified in an exam question; Na 2 CO 3 (s) + 2HCl (aq) → 2NaCl (aq) + . The lower detection limit of gas volume was estimated to be ~0.5 μL assuming that the smallest bubble size that can be detected is about 500 pixels in size. Additional InformationThe volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure (Boyle’s law), Charles and Gay-Lussac demonstrated that the volume of a gas is directly proportional to its temperature .
how to measure gas produced
The resulting statistical analysis of this sample size produces the "average" behavior (i.e. velocity, temperature or pressure) of all the gas particles within the region. . the trapped gas' volume decreased (this is known as an inverse relationship). Furthermore, when Boyle multiplied the pressure and volume of each observation, . From the analysis in Section 4.2 it is clear that there is a strong positive correlation between off-gas volume and battery capacity across the entire range from Wh to kWh. However, the amount of gas produced specific to battery capacity is .
Conclusion. Gas-based absorption methods, such as the BET method for scanning the surface area and the BJH tool for pore size distribution, are essential for material identification. These surface area and pore size analysis methods furnish important information about the surface characteristics and porosity of materials that are crucial for developments in fields that include .
gas is supplied as a purge gas in the column connection part, or a taper is formed in the lower part of the insert so that the wide bore column closely adheres to the bottom part of the insert. When using a septum purge type total volume injection port, it can be used with a carrier gas fow rate near the optimum fow rate (about 5 mL /minVolumetric analysis, any method of quantitative chemical analysis in which the amount of a substance is determined by measuring the volume that it occupies or, . compound is burned in a furnace under conditions that ensure the conversion of . Figure 1: A simplified diagram of a gas chromatograph showing: (1) carrier gas, (2) autosampler, (3) inlet, (4) analytical column, (5) detector and (6) PC. Credit: Anthias Consulting. After injection into the GC inlet, the chemical components of the sample mixture are first vaporized, if they aren’t already in the gas phase.
With more than 25 years of industry experience specializing in natural gas measurement, volume correction, and data acquisition, he adds his voice to the Linc Energy Blog by writing about natural gas flow measurement and control. Jeff has been in a . The volume of the evaporated gas will reach several hundred times the volume of the liquid (Bubbico et al., 2018). Ignition factors are highly likely to occur in such a wide range of spaces. . In addition to the use of traditional exploration technology, it is extremely critical to perform numerical analysis of the gas explosion process in .
how to measure gas mass
gas volume measurement units
10 de mar. de 2023 · When Tisca reveals that Napoleon has offered her a job, Ramesh gets upset that his children are taking decisions independently. Mansukh suggests a family .
gas volume analysis|measure volume of hydrogen gas